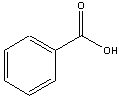
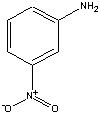
| Benzoic Acid | m-nitroaniline1 | 2-methoxynaphthalene |
|---|---|---|
 |
 |
 |
| 1para or meta-nitroaniline may be used for this experiment | ||
This experiments allows you to separate a solid-phase mixture consisting of (i) 2-methoxynaphthalene, (ii) m-nitroaniline, and (iii) benzoic acid using Separatory Funnel extractions. Since each of these chemicals have similar solubilities in water and ether, you can effect a separation their chemical properties. For example, benzoic acid is insoluble in water but the benzoate ion is soluble in water. Therefore, under neutral conditions, the benzoic will partition into the ether layer. Adding NaOH will neutralize the benzoic acid producing the benzoate ion, which now goes into the aqueous layer, leaving other other two organic compounds in the ether. Likewise, nitroaniline is not soluble in neutral or basic conditions, but is soluble in acidic solutions. Making the liquid in the Separatory Funnel acidic will allow the nitroaniline to be positively charged, and it will now partition into the aqueous layer. Isolating the aqueous layers allow you to crystallize the benzoic acid and nitroaniline as describe in this protocol. Final analysis of the isolated chemicals will be done using melt point analysis. You will also need to determine a percent yield for each.
| Benzoic Acid | m-nitroaniline1 | 2-methoxynaphthalene |
|---|---|---|
 |
 |
 |
| 1para or meta-nitroaniline may be used for this experiment | ||
In Experiments Ia and Ib you purified a solid by selecting a solvent that a particular chemical is soluble in, but which another remains insoluble (e.g., acetone dissolved aspirin but not the NaCl or silica) and by using the method of re-crystallization you were able to isolate benzoic acid in pure form away from contaminants. However, there are many times when neither of these methods are be appropriate because you may need to isolate two or more solids from the same mixture that have very similar physical or chemical properties. For example, and both solids are organic in nature, with similar solubility properties, or when the liquids you are trying to separate boil at nearly the same temperature (distillation is a technique we will use in a later experiment). Solvent extraction is a procedure that will work well in many of these cases. During an extraction, one solvent is used to dissolve (or partition) one or more of the components of the mixture, leaving other components behind (in the solid phase, the liquid phase, or in another solvent). In this experiment we will be working with "liquid-liquid" extractions, but the general principles will be the same for "solid-liquid" extractions as well.
Zubrick makes the important distinction between "extraction" and "washing". While the same series of experimental steps are generally used to carry out both of these techniques, and they use the same theory, the main difference is that in an extraction you are moving a substance you want to keep into a new solvent, while in washing you are moving a substance you will throw away into a new substance. In future experiments you may be instructed to "extract the product mixture with 3, 10-mL portions, of ether," or "wash the ether extracts with 10% sodium bicarbonate, and then with water." These instructions assume you understand the difference between extraction and washing.
In this experiment you will be given a mixture containing 2-methoxynaphthalene, m-nitroaniline and benzoic acid. The first step in the separation will be to dissolve the entire mixture in one organic solvent (diethyl ether). You will use the chemical and physical properties of these compounds to determine how to separate them in pure form using extractions (and washings, where necessary), and isolate the m-nitroaniline and benzoic acid in solid form. Follow the guidelines given in chapter 15 of Zubrick in determining the solvents you will use, the order in which you perform the extractions, and the methods you will use to get each compound back into the solid form.
Safety
To do the experiment, obtain about 3 grams (2.5-3.0 grams is acceptable) of the solid mixture consisting of approximately equal portions of:
You will be separating from the above mixture pure benzoic acid and pure m-nitroaniline.
Under normal conditions, all three of your organic compounds will dissolve in the organic layer (the diethyl ether), not the aqueous layer. The only way to separate the organic compounds from each other is to rely on their chemical properties, not just physical properties. To this end, you should always remember that neutral organic compounds do not dissolve to a great extent in aqueous solutions. Alternatively, charged organic compounds will often dissolve in aqueous solvents because they are ions. Conversely, neutral organic compounds dissolve in organic solvents (e.g., diethyl ether) whereas charged organic compounds will not dissolve (or stay dissolved) in organic solvents.
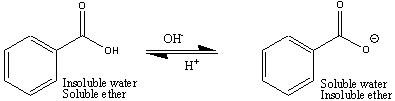
It is possible to force an organic compound, that is charged, out of the organic phase into the aqueous phase. This process is usually performed using a Separatory Funnel. When a compound leaves one phase and goes into the other phase, this process is referred to as partitioning. For example, when the benzoate ion, derived from benzoic acid, leaves the ether phase and goes into the aqueous phase, we refer to this as partitioning into the aqueous phase.
The figures below show how this is done in order to isolate different compounds based on their acid-base properties. Benzoic acid is soluble in ether, but when neutralized and converted into benzoate ion, it is now soluble in water, so will partition into the lower aqueous phase, leaving uncharged organic compounds in the ether. Once the aqueous layer is isolated, benzoic acid is easily isolated when the benzoate ion is converted back into benzoic acid following the addition of 6 M HCl.
m-nitroaniline is soluble in ether, even when the aqueous layer is basic, because a base stay base in base. However, when a solution of 1 M HCl is added to the Separatory Funnel, the amino group accepts a proton which produces a charged molecule. This charged molecule then partitions into the aqueous layer, and can be collected. When the aqueous layer has 6 M NaOH added to you, the m-nitroaniline is regenerated, and since it is now insoluble in water, you can collect it as a solid.
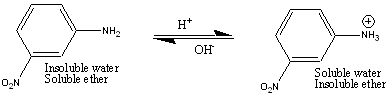
The general rule you must remember is that uncharged organic compounds stay in the organic (e.g., ether) layer. Charged organic compounds partition into the aqueous layer. It doesn't matter whether the organic compound acquires a positive or negative charge, it will partition into the aqueous layer, out of the organic layer. Acids (i.e., benzoic acid) are converted into their negatively charged ions in the presence of base, and partition into the aqueous layer. Bases (e.g., m-nitroaniline, which is a base because it is a proton acceptor) become positively charged when reacted with an acid, and this positively charged ion will now partition into the aqueous layer. Charged molecules partition into the aqueous layer and uncharged molecules partition into the organic layer.
Using a powder funnel (a plastic funnel with a wider opening and that fits in the ground-glass opening of your Separatory Funnel) add all of your mixture to the Separatory Funnel. Add the following to the Separatory Funnel:
By adding the NaOH to the organic compound mixture (with diethyl ether), you will convert the benzoic acid (uncharged) into sodium benzoate (negatively charged), which is now soluble in aqueous solutions. The other organic compounds, which stay neutral in basic solutions stay uncharged in the diethyl ether layer. The negatively charged benzoate ion now partitions into the lower aqueous layer when you shake the contents of the Separatory Funnel. Be certain that you thoroughly mixture the contents of the Separatory Funnel to allow effective partitioning of the benzoate ion into the aqueous layer.
Once you see the appearance of two distinct layers in the Separatory Funnel (upper organic layer and lower aqueous layer), you can collect the lower aqueous layer which contains the benzoate ion. To collect the lower layer, first remove the ground-glass stopper, and then open the stopcock valve. Let the lower aqueous layer drain out until the top organic layer just reaches the stopcock value opening. Close the stopcock valve, and prepare to isolate the nitroaniline.
In order to collect your benzoic acid, you need to convert the benzoate ion (which is completely soluble in water) back to benzoic acid (which is mostly insoluble in water). To effect this conversion, you must first add acid to the benzoate solution, which will then allow benzoic acid to form.
Using the 6 M HCl solution add enough acid to convert all of the benzoate ion to benzoic acid. As a first approximation, you will need about 5 mL of the 6 M HCl to neutralize your solution. Keep adding 6 M HCl until the solution is acidic, based on litmus paper. When you think you having an acidic solution add about 2 mL more 6 M HCl, because you cannot have too much acid. A little phrase you can remember is: "acid stays acid in acid."
Collect your solid benzoic acid above using vacuum filtration with a typical Buchner Funnel filtration setup. For this particular experiment, do not store the filter paper in the oven as it will decompose at a high temperature when the paper is acidic. So, just scrape your solid benzoic acid into an evaporating dish (or 100-mL beaker) and place it in the drying oven until the next lab period.
After you have removed the benzoic acid from the ether layer (by the addition of NaOH above), you are left with an ether layer still containing the nitroaniline and methoxynaphthlene. In order to effect the removal of the nitroaniline, you rely on the fact that nitroaniline functions as a Bronsted-Lowry base (proton acceptor) much like how ammonia (NH3) accepts a proton to become the ammonium ion (NH4+). In the case of the nitroaniline accepting a proton, it becomes the nitroanilinium ion. Therefore, when you add a solution of 1 M HCl to the ether layer, you convert the neutral nitroaniline into the charged nitroanilinium ion, which can then partition into the acidic aqueous layer.
Add 30 mL of the 1 M HCl solution to the Separatory Funnel. Following this addition, replace the ground-glass stopper
Caution: After using ether, keep the lid on the ether bottle when not in use.
Dissolve the solids in the separatory funnel by adding the stopper and mixing thoroughly. You can do your separatory funnel extractions in the lab (outside of the hood), but any open containers with ether must be handled in the hood (you can remove the stopper to the separatory funnel in the lab without problem).
Using known chemical and physical properties for these compounds develop an extraction scheme in order to separate the three solids, and to isolate each compound in solid form. For example, what are the physical properties of these compounds, what are their solubilities or their lack of solubility in certain solvents (e.g., in ether or aqueous solvents). The extraction and washing solvents available are: water; 10% sodium bicarbonate solution; 6 M sodium hydroxide solution; 6 M hydrochloric acid solution. Use 6 M HCl or 6 M NaOH to neutralize your aqueous solutions collected containing either the benzoate ion (extraction in base) or the nitroanilinium ion (extraction in acid) in the aqueous solutions.
Once you have successfully extracted either the m-nitroaniline or benzoic acid into one of the aqueous extraction solvents, you will need to isolate that chemical in pure solid form. All of the organic chemicals will be initially soluble in the organic solvent (diethyl ether). For example, how can you force the benzoic acid to be partitioned (extracted) into one of the extraction or wash solutions for purification? Once it is in the appropriate solution as a dissolved solute, how can you convert it into an insoluble solid to isolate and characterize?
After you have isolated both the m-nitroaniline and benzoic acid in pure forms, you can discard the remaining organic liquid, containing the 2-methoxynaphthalene in the organic liquid waste container.
How much m-nitroaniline did you recover? How much benzoic acid did you recover? What are the physical properties of these compounds that you observed (color, crystal shape, etc.)? What is the melting point for each these compounds? How do these values compare to the literature values? Do you think that you isolated these compounds in pure form, based on the observed melting points?
Read chapters 15 and 16 in Zubrick.
| Acids | Uses of acid solution | Base | Uses of basic solutions |
|---|---|---|---|
|
6 M HCl |
Use this solution only to neutralize a basic solution never for extractions | 6 M NaOH | Use this solution only to neutralize an acidic solution never for extractions |
| 1.0 M HCl | Use this solution for washes and extractions (e.g., in a separatory funnel) never for neutralizations | 1.0 M NaOH | Use this solution for washes and extractions (e.g., in a separatory funnel) never for neutralizations |
| 10% NaHCO3 (sodium bicarbonate solution) can be used to neutralize an acidic solution within a separatory funnel, if you want to end up with a solution near neutral pH. To get rid of the NaHCO3, simply use 2-3 DI water washes, effectively washing the ether layer and getting rid of the bicarbonate ion. Do not use sodium bicarbonate solution to neutralize an acidic solution not in a separatory funnel. | |||
Litmus paper is an effective and inexpensive method to determine if a solution is either basic or acidic. Use litmus paper in this experiment to determine if you have produced an acidic or basic solution.
|
|
| Compound | MW | Amount | mmol | mp | bp | Density | ηD | msds |
|---|---|---|---|---|---|---|---|---|
| Benzoic Acid | 122.12 | --- | --- | 122.4 | 249.4 | 1.2659 | --- | view |
| m-Nitroaniline | 138.12 | --- | --- | 114 | 331.7 | 1.424 | --- | view |
| 2-methoxynaphthlene | 158.2 | --- | --- | 73-75 | 274 | n/a | --- | view |
| Diethyl ether | 74.12 | 30 mL | --- | -116.3 | 34.6 | 0.7134 | --- | view |
| HCl | view | |||||||
| NaOH | view | |||||||
| Compound | g/mol | grams or mL | 10-3 M | oC | oC | g/mL | ηD | msds |