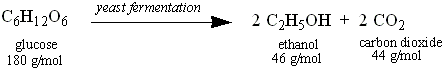Experiment 5
Liquids: Distillation, Boiling Points, and the Fermentation and Distillation of Ethanol
Objectives
Distillations are performed to isolate volatile chemicals from non-volatile
or less volatile chemicals. This experiment addresses how distillations (both
simple and fractional) can be used to isolate chemical in pure form. In
addition, boiling points and other physical properties can be determined,
including refractive index and density of purified materials. We also setup a
fermentation to isolate ethanol from these mixtures.
Background
Parts A and B will be performed during the first laboratory period.
Part C, which is the distillation of ethanol from a
fermentation mixture, will be performed during the second laboratory
period.
Techniques and procedures that you will perform during this
experiment include:
-
Simple distillation
-
Fractional distillation
-
Refractive index determination
This experiment is performed in three parts over two days.
Parts A & B are performed during the first day.
Part C is performed during the second day of lab.
The fermentation setup is done one week prior to the
beginning of Part C.
Part A:
Purify 2-propanol by simple
distillation. This technique shows how to purify, by distillation, a
volatile organic compound. This procedure is good for
purification of volatile compounds from compounds that are not
volatile, but not very successful for mixtures of compounds, each of
which is volatile. Part B describes how to fractionate these
types of mixtures using fractional distillation.
Part B:
Separate ethanol from water
by fractional distillation. Compounds which are volatile will
each distill at temperatures well below their actual boiling points
(e.g., remember the vapor pressure of water?). We need to
have a procedure that allows for more efficient separation of these
volatile liquids, and this is the process of fractional distillation.
Part C:
We will prepare a
fermentation of glucose to produce ethanol. The ethanol from
this fermentation will be isolated by fractional distillation (using
the procedure described in Part B). You must
repeat your fractional distillation, in order to remove water, which,
unfortunately predominates during the first fractional distillation.
Once you have repeated your fractional distillation, you can
determine the amount of alcohol in your final distillate sample.
In many of the previous experiments that we have performed, (e.g., the
experiments involving extraction, separation, Re-crystallization, and
chromatography), you have learned that a chemist must be able to
exploit the specific physical properties of the components of a mixture
in order to efficiently separate and purify the desired chemical
compound. The most common method for separating and purifying volatile
liquids is distillation, which makes use of the specific boiling points
of the liquid components in the mixture.
When there is only one volatile liquid, or when one of the liquids has
a boiling point well below the others, a simple distillation can be
used. However, if there are two or more liquid components, which have
boiling points near each other, a fractional distillation must be used
(the theory of distillations is discussed in Chapter 35 of Zubrick).
In Part A of this experiment you will carry out a simple distillation
of 2-propanol (isopropyl alcohol), which has been contaminated with a
non-volatile impurity (a dye). In Part B, you will carry out a
fractional distillation of a 50:50 (v/v) mixture of ethanol and water.
During each of these experiments, you will determine the boiling point
of the volatile organic compound during distillation. You will monitor
the temperature that the volatile liquid has, in the gas phase using a
thermometer. This temperature should remain constant, and
should reflect the actual boiling point of the chemical under the
atmospheric conditions of the day. Report this boiling point as part of
your data.
Procedure
Part A: Simple Distillation of 2-propanol
Safety:2-propanol is a highly flammable liquid and a severe eye irritant -- no
flames will be allowed in lab while it is in use. As for every
experiment, goggles must be worn, even though you may not actually be
working the chemicals, if there is anyone using 2-propanol in
the lab.
Follow the instructions in chapters 19 and 20 of Zubrick for setting up
a simple distillation apparatus, although the instructor will go
through the setup, and the use of your organic kit. You will
use a 50-mL round bottom flask as the distillation pot, and a 100-mL
round bottom flask as the receiver. As a standard rule,
anytime you are boiling an organic compound, you will always include a few
(not a handful!) boiling stones to keep the solution boiling smoothly!
Using a beaker (not a graduated cylinder) obtain about 30-40 mL of "impure" 2-propanol (this sample of 2-propanol
has had a small amount of a soluble, non-volatile dye added to it as an
impurity). Add the 2-propanol to the distillation pot (never pour anything through a ground-glass opening without using a funnel), add the boiling
stones, and begin the distillation (remember to turn on the cooling
water before you turn on the heat). Collect your distillate in a pre-weighed graduated cyclinder (10-mL or 25-mL or 50-mL cylinder).
Once the temperature starts to rise
above room temperature, you should start to record the thermometer
reading every minute.
You
should start to record the temperature at any time up to when the solution starts to boil. Record every minute the temperature you read. Continue to do your distillation until you have collected about 20 mL of distillate. Be sure that the distillation pot never goes dry (never
let a heated flask go dry!).
You must
plot your data by
hand using graph paper, or you can use Excel or another graphing program for a graph for inclusion in your lab notebook and written report.
Measure the volume of the distillate collected. Using a pre-weighed graduated cylinder (10-mL or 25-mL or 50-mL cylinder), you should determine the density of the collected distillate. You will also determine the refractive index of your distilled liquid. Your
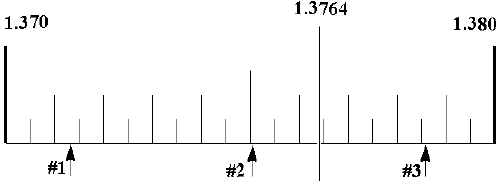
instructor will describe how a refractive index is determined.
Dispose of your liquid, and any liquid remaining in the distillation
pot, in the liquid waster. Be sure to make certain that no
boiling stones are deposited into the liquid waste. Put the
boiling stones in the solid waste container.
Part B: Fractional Distillation of an Ethanol/Water Mixture
Safety: Ethanol is a flammable liquid and an irritant; avoid contact and
inhalation -- wear gloves while handling it. No flames will be allowed
in lab while ethanol is in use. Goggles must be worn whenever
anyone is using chemicals.
Set up a fractional distillation apparatus as demonstrated by your
instructor. Use glass beads to pack the
fractionating column (your instructor will demonstrate how to pack the column). Try
adding some glass beads directly to your fractionating column. If the
glass beads stay in the column, there is no problem, but if any beads go
through, try adding a larger amount of glass bead, and their packing inside the
fractionating column should allow them to stay in place. Do not ever use glass
wool or anything besides beads in the fractionating columns.
To
do this part of the experiment, you will use a 100-mL round bottom
flask as the distillation pot. You will need a number of receivers; it
is best to use test tubes. Measure into one test tube about 4 mL of
water. Use this sample to know how much liquid you need to obtain about
4 mL of distillate during this part of the experiment. Continue
collecting 4-mL samples until you have collected about 30 mL of
distillate. Determine the refractive index of each collected sample, as
well as determining the refractive index for pure ethanol and pure
water.
-
Obtain about 50.0 mL of the 50% (v/v) ethanol/water mixture, and pour
into the distillation pot.
- Add
a few boiling stones.
- Turn
on the heating mantel to obtain a steady boiling mixture.
- Monitor
time and temperature during the entire distillation process
Start
recording the temperature as soon as your sample begins to boil. Record
the temperature every 30 sec. Collect your distillate into test tubes.
You should collect about 4 mL in each test tube, but it is not
necessary to measure each tube. As a comparison, add about 4 mL of
water into a test tube. Collect about the same amount of liquid into
each of the tubes during the distillation process. Continue recording
the temperature until you stop collecting your samples. Collect about
30 mL of distillate.
You
must plot your data by hand using graph paper, or you can use
ChemWorks™ on the computers for inclusion in your lab
notebook and written report. You should have two plateaus, one for the
boiling point of the ethanol and the other for the boiling point of the
water. Your graph for your lab report and for your notebook must show
these two plateaus.
After the distillation has finished, you will have a good determination
for the boiling point of ethanol. Determine the refractive
index for each of your samples.
Do not ever throw any glass beads away. At an expense of about 25 cents
($0.25) per glass bead, they are very expensive. Keep your glass beads in
your fractionating column (add some tissue to the top to prevent spillage) until
the next lab period. Never throw away any glass beads.
Part C: Fermentation and Distillation of Ethanol
Yeast
ferment sugars to produce ethanol. You will use glucose (dextrose) as
the sugar the yeast will use for alcohol production. Glucose has a
molar mass of 180 g/mol. The molar mass of ethanol is 46 g/mol. The
other product of fermentation is carbon dioxide, which we will not
consider in the current experiment. The equation for this reaction is
as follows:
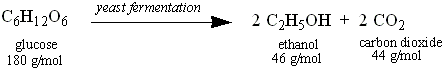
Based
on the balanced equation, one (1) mole of glucose will yield two
(2) moles of ethanol. Based on these molar ratios, one mole of glucose
is 180 g, and two moles of ethanol is 92 grams. On the other hand, one mole of sucrose will yield four moles of ethanol. Your Instructor will inform you which carbohydrate you will be using.
- How
many grams of alcohol would you produce, if you started with 60.00
grams of glucose (MW=180.0)?
- If
you started with 60 grams of sucrose (MW=342.3), how many grams of ethanol would
you produce?
Based
on the amount of carbohydrate (glucose or sucrose) you actually started with (based on the amount you weighed out), what is the
theoretical yield of alcohol that you should produce? Use this value to determine your percent
yield after you have finished this part of the experiment.
|
Grams of glucose (or sucrose)
fermented: _________________ g |
Theoretical yield of
ethanol: __________________ g |
Fermentation Setup
Set up the fermentation container in the following manner:
- Place
60.00 g (0.33 mole) of glucose (or of sucrose) into a 250-mL Erlenmeyer flask (or the
"beer-bottle fermented")
- Add
175 mL of distilled water
- Add
20 mL of the Pasteur's salts solution (see
Footnote 1 for formulation)
- Add
2.00 g dry yeast, that has been rubbed
to a thin paste (to break up
yeast clumps) with about 10 mL of DI water
- Shake
vigorously to mix the contents of the fermentation container
(containing all reagents)
- Close
the container with a rubber stopper attached to a piece of glass tubing
connected by a piece of latex tubing. The glass tubing at the end of
the latex tubing will be inserted into a tube containing water (2-3 mL DI water) and a
small amount of mineral oil (pour a little mineral oil to cover the water in the test tube) to prevent evaporation.
Allow the fermentation mixture to stand at a temperature of 25-35oC
until fermentation is complete (about a week is required).
First Distillation of Fermentation Mixture
After the fermentation is complete, you will distill the alcohol from the
liquid mixture, including the solid, particulate material that is not
soluble. Gently pour all of the liquid into a 500-mL round-bottomed
flask (checked out from the stockroom). The liquid will be
somewhat cloudy, but this will not interfere with the first
distillation (do not ever let a distillation flask go dry, but this
should be of little concern here since we will only collect a fraction
of the liquid via distillation).
Add
a few (6-8) boiling stones to the flask. Attach a fractional
distillation column (filled with glass beads, as described in Part
B) to the top of the
distillation flask, and attach the still head and condenser unit. You
do not
need to monitor the temperature as the first distillate is mostly
water, so the temperature will be about 100oC.
Collect about 50 mL of distillate into a pre-weighed graduated
cylinder (a 50- or 100-mL graduated cylinder can be used).
After
the first distillate has been collected, observe and record the
collected volume, and weigh the graduated cylinder to determine the
mass of collected distillate (total mass of cylinder containing the
distillate minus the mass of the empty graduated cylinder).
You should now calculate the density of the distillate, based
on the mass of the distillate you just determined divided by the volume
collected. From the Table of Densities of aqueous ethanol
solutions, given in Footnote 2, calculate the mass of alcohol collected
in this first distillate.
To make your determination of the amount of ethanol isolated, you can estimate
your percent alcohol using the table at the end of this experiment. This table shows you several ways to determin the amount of ethanol in your sample based on density. The easiest column to use is the first column which shows the mass percent of alcohol (% by mass) as follows:
Using
the density of your distillate from the above calculations, determine
the percent alcohol ( you may have to extrapolate (e.g.,
Calculate
the percent yield of ethanol produced in the fermentation, based on the
theoretical amount of ethanol that would be produced from the starting
amount of glucose (each glucose molecule produces two ethanol
molecules). This "crude" distillate will be used in a second
fractional distillation.
An additional table is available, using an interpolative procedure to determin percentages which do not fall within the larger percent range shown in the table below (percentages are in 5% increments). The new table (which is an Adobe pdf document) shows you how to get to the percent values, based on density, in unit values, e.g., 45%, or 46%, or 47%, etc. The highlighted values in this pdf document shown how to determine percent concentration values between 45% and 50% ethanol. You can download and print this document at this URL of a pdf document.
Discard the residue left in the distillation pot, which contains mostly
water.
One can obtain 95% ethanol (but not 100% ethanol; do you know why? What
is an azeotrope?) from the dilute alcohol mixture obtained during the
first distillation. For the second distillation, monitor the
boiling point carefully, as you should collect the material that
distills at a temperature of 78-82oC;
if too little distillate is obtained in this range, continue the
distillation and collect the fraction boiling at 82-88oC.
Prior to doing your second distillation, empty the glass beads
from the fractioning column into a beaker you will wash them yourself with soap
and water, and then reuse them. Also, you need to wash with soap and water each of the following pieces of glassware:
- Fractionating column
- Still head
- Condenser column
You can now re-assemble your distillation setup for a fractional
distillation, and then follow the procedure for collecting and analyzing your
second-distillate liquid.
At the end of the experiment, put the used glass beads into a
beaker on the Instructor's bench to be washed by the Stockroom Personnel. Do not
throw the glass bead away.
Procedure
for collection of ethanol via a second distillation:
- Add
the alcohol-water mixture from the first distillation (after weighing and determining
its density) to a 100-mL round bottom flask.
- Add
a few boiling stones to your round-bottom flask (distillation pot) to maintain a slow, steady boiling.
- Start
monitoring the temperature as soon as you turn on the heat, or at least prior to the solution boils. Monitor the
temperature at regular intervals, usually every minute, until you stop collecting your samples.
- Start to collect
4-mL samples (using you conical vial in the organic kits or a test tube) until the temperature rises significantly above the normal 78-82oC temperature,
as described above. It is still permissible to collect above the 82oC degree range, but your samples contain more and more water. However, if you have not collected at least 4-5 samples, each of about 4-5 mL volumes, continue to collect samples (regardless of temperature) until at least 20 mL of solution is collected. Based on theoretical yeields, and actual lab experience, you could have produced 30-36 mL of ethanol during your fermentation, so collectin at least 20 mL is not out of reason.
- Do not determine a refractive, but you must determine a density for each sample. To do this, collect about 4-5 mL in a conical vial (as mentioned above), and then immediate transfer the contents of the vial into a pre-weighed 10-mL graduated cylinder. After transfer of collected material into the 10-mL graduated cylinder, reattach the vial to the vacuum adapter, and collect another 4-5 mL sample.
- Determine the mass and volume of the sample in the 10-mL cylinder to calculate a density of the sample. After determining the mass and volume, transfer its contents into a pre-weighed 50-mL graduate cylinder to collect all samples for an overall density and yield of ethanol for the entire experiment.
- After you have analyzed individually the 4-mL samples, combine
your 4-5-mL collected distillate samples into the 50-mL graduated cylinder, the total volume, the mass of the sample in the 50-mL cylinder, you can determine the density.
- From the density just determined (step #7), determine the total mass of ethanol collected during the second fractional distillation. No need to determine a refractive index as density (and total mass of solution) will be our criterian for yield.
- Determine
percent yield based on the overall yield from step #8, by dividing the actual yield by the theoretical yield times 100 to get a percent yield.
Based
on the amount of collected alcohol, its density, what is the percent yield? What is the theoretical yield?. Your
instructor will help you determine the percentage of alcohol based on
your refractive index and density determinations.
Do not throw any glass beads away. At an expense of about 25 cents
($0.25) per glass bead, they are very expensive. After all distillations
are completed, pour your glass beads into a large beaker on your instructors
bench, so they can be cleaned. Never throw glass beads away.
Chemicals and Reagents
| Compound |
MW |
Amount |
mmol |
mp |
bp |
Density |
ηD |
msds |
| 2-propanol (isopropyl alcohol) |
60.1 |
50 mL |
|
-88.5 |
82.5 |
0.78505 |
1.3772 |
msds |
| Dextrose (glucose) |
180.16 |
50.0 g |
277.53 |
146 |
--- |
1.54 |
|
msds |
| Sucrose |
342.3 |
50.0 g |
146.07 |
160-186 |
--- |
1.59 |
|
msds |
| Ethanol |
46.0414 |
|
|
-114.1 |
78.5 |
0.8 |
1.3614 |
msds |
| Compound |
g/mol |
grams or mL |
10-3 M |
oC |
oC |
g/mL |
ηD |
msds |
Copyright © Dr. Donald L. Robertson (Modified:
11/14/2012)