For most of the experiments we have performed this semester, we have set out to convert one chemical into another with various different methods. For a synthetic organic chemist the product of one reaction is often used as the reactant in another. This is also the situation that occurs in biochemistry where different chemical pathways use a newly synthesized product to be a reactant for the next reaction in that pathway. This is particularly important for the current experiment because the first chemical transformation occurs using a vitamin as the "co-enzyme" for the synthesis of benzoin. While enzymes are normally considered to the biological catalysts in cells, it is often a co-enzyme, derived from a vitamin, that actually performs the biochemical transformation. Thiamine, vitamin B1, will be used in the current experiment to convert benzaldehyde into benzoin. The benzoin produced in the first experiment will then be used in the second to produce benzil. Likewise, the benzil will then be used to synthesize benzilic acid. Hence, a multi-step synthesis of different compounds. These new compounds can then be used in various different applications, some of which are for medicinal purposed. The student can research their ultimate uses to observe that no chemical synthesis is for naught. The last experiment requires the student to devise a method to convert benzaldehyde into benzoic acid, perhaps employing oxidation methods used previously, or use a method that might be new to the student, even something not previously discussed during lecture or lab. Therefore, to provide a variety of methods for producing benzoic acid, students should work independently, examining general oxidation methods for converting aldehydes into acids obtained searching the chemical literature.
This multi-step experiment is divided into four sections:
Each part will be performed on successive days. The product from the previous day's experiment will be used as the reactant on the next day's experiment. It is vital that you do the experiments on the days they are described. Part 4 is a "self-designed" experiment where you devise a protocol to follow to prepare and purify benzoic acid from benzaldehyde. You must design this experiment, and get the approval of your instructor. You must then make a list of reagents you will need to fulfill this experiment.
There are five laboratory periods devoted to this experiment. The first four lab periods are used to make the chemicals listed for those days. The fifth day can be used to finish up any incomplete analysis, including melt points, yield, etc.
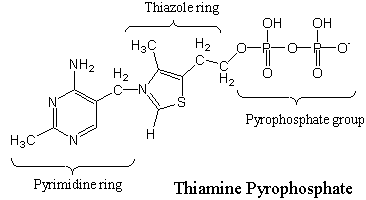
Vitamin B1, thiamine, as its pyrophosphate derivative (shown below), is a coenzyme universally present in all living systems. It was originally discovered as an essential nutrient required to prevent the human disease beriberi, which affects the peripheral nervous system. Symptoms include pain and paralysis of the extremities, emaciation, or swelling of the body. The disease is still common in the Far East.

In biochemical terminology, thiamine functions as a coenzyme, a biological molecule that assists in enzymatic reactions. In most cases, coenzymes are directly involved in the biochemical reaction that the enzyme catalyzes since they usually bind the substrate (reactant) for the reaction. Without the coenzyme, no reaction will take place. The enzyme, which is the biological catalyst, binds the substrate, controlling stereochemistry, energetics, and entropic factors. As indicated above, the vitamin (derived from VITal AMINe) thiamine is required for many enzymatic reactions. In the current experiment, we will use thiamine to catalyze the reaction of benzaldehyde into benzoin.
Thiamine, functioning as a coenzyme, can be used for (1) the non-oxidative decarboxylations of a-keto acids, (2) the oxidative decarboxylations of α-keto acids, and (3) the formation of α-hydroxy ketones. Most biochemical processes are no more than organic chemical reactions carried out under special conditions. Like most reactions in organic chemistry, many biochemical reactions can now be explained using familiar reaction mechanisms. To enhance reactivity, and to be stereoselective, enzymes are used to bind the substrate in a manner that allows only a single reaction, with stereoselectivity to occur. In addition, enzymatic reactions can be carried out in mild conditions and at moderate pH values. Reactions which involve hydrophobic (lipid loving or water hating) conditions that might not otherwise be possible in an aqueous, biological environment.
Part 1 of this experiment is designed to illustrate these types of processes. As a biological reagent, the coenzyme thiamine in this reaction will be used to carry out an organic reaction chemistry reaction without using an enzyme. The reaction is an acylion condensation to benzaldehyde (as an example, if you have 10.00g of benzaldehyde that you start with, seeing that it takes two moles of benzaldehyde to produce one mole of benzoin, what is the theoretical yield of benzoin?):

From a chemical point of view, many coenzymes have what we call "a business end" to the molecule and the rest of the molecule. The reactive part of thiamine is the thiazole heterocyclic ring (a 5-membered ring containing both a sulfur [thio] atom and a nitrogen [azo] atom). This ring is the reactive portion of the coenzyme. The rest of the molecule is important biochemically for enzyme associate, etc., but the thiazole ring is the reactive portion. The rest of the molecule is important biochemically, but it is not required for the reaction described here.
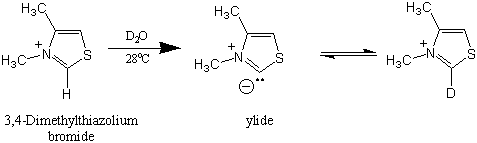
Experiments with the model compound 3,4-dimethylthiazolium bromide have explained how thiamine-catalyzed reactions work. Part of the chemistry of thiamine is the acidic proton located on the carbon between the sulfur and nitrogen atoms. Using 3,4-dimethylthiazolium bromide, it was found that there is a rapid exchange of the C-2 proton for deuterium in the D2O solution. At a pD of 7 (No pH here!), this proton was completely exchanged in seconds!
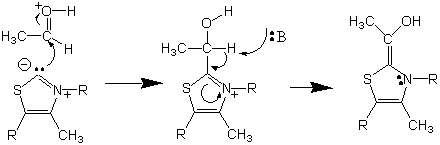
The enamine which we will produce, using benzaldehyde, can react with a second benzaldehyde molecule to produce the desired product, following the acyloin condensation pathway. The enamine functions much like the enolate partner in an acid-catalyzed aldol condensation. It can condense with a suitable carbonyl-containing acceptor to form a new carbon-carbon bond. Decomposition of the intermediate to regenerate the thiamine ylide yields the protonated acyloin, benzoin, in this reaction. The final product depicted below just needs to undergo deprotonation to produce benzoin.
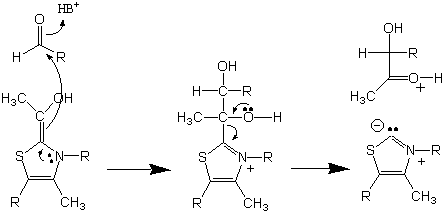
The reaction pathway outlined above describes the pathway we will follow to produce benzoin using thiamine as catalyst. The benzoin produced will be used for the sequence of reactions which will be followed to produce benzil and benzilic acid.
In this, the first step of our multistep reaction sequence (performed during day one), benzaldehyde will be condensed, using the thiamine as a coenzyme catalyst, to produce benzoin. The same reaction can be performed using the cyanide ion (you should include a plausible mechanism for cyanide catalyst and include it with your report). The reaction mechanism for thiamine catalysis is described above. (It is imperative that the benzaldehyde be from a newly opened bottle because of the ease of oxidation, producing benzoic acid, which will interfere with the reaction. The thiamine hydrochloride must also be from a newly opened bottle, although it is not quite as critical as for the benzaldehyde.)
The overall reaction for conversion of benzaldehyde into benzoin is:

Benzoin is made following using the chemicals listed below:
Heat the mixture at 60oC (using a constant temperature water bath set to 60-65oC) for about 1.5 hour. However, an easier and more straightforward procedure is to heat the sample at 60oC for 15 minutes after reaching this temperature (allow about 5 min for warming the sample before starting to record the time, then incubate for the 15 minutes; going long is not bad.)
Caution: The temperature of this reaction cannot go above 65oC. Constant monitoring of temperature is paramount during this part of the reaction and must be maintained between 60-65oC.
As mentioned above, you can let the reaction procedure for the full 90 minutes. However, the following alternative incubation should be used, since it only required about 20-30 minutes of total incubation time. The 1.5-hour reaction described above could still be used, but the shorter time is preferred. Therefore, incubate your reaction mixture at 60-65oC for at least 15 min (allow five minutes to equilibrate temperature). After this minimal incubation time, you will store the reaction mixture until the next lab period (for at least 24-48 hours) at room temperature. The Erlenmeyer flask containing the reaction mixture will be sealed using a regular cork to seal the flask. At the beginning of the next lab period, collect all of your solid material using vacuum filtration as normal. (Remember that the rate of reaction usually doubles for every 10oC increment in temperature.)
At the beginning of the next lab, retrieve your reaction flask from the reagent cart. You should be able to observed crystals, which are a slight yellow in color. If crystals did not form after storage, withdraw a drop of the solution on a stirring rod and let it dry to produce a solid on the glass rod; then, rub it against the inside surface of the flask to induce crystallization.
Collect your crystals via vacuum filtration (wash it free of the yellow mother liquor with a 1:1 mixture of 95% ethanol and water; prepare by mixinf 20 mL of 95% ethanol with 20 mL of DI water). Since crystals will be moist you cannot do a yield and melt temp. However, your sample should be dry enough to proceed to the next experiment making benzil.
Optional: Remember, only if your instructor instructs you to perform a re-crystalization, then use the following procedure, but only if your instructor wants you to do this re-crystallization. In most cases it is not necessary, just proceed with the Part 2 experiment.
Dispose of the liquid filtrates in the liquid waster container.
Starting with the α-hydroxyketone benzoin (prepared in Part 1), you will prepare an oxidation product, benzil, which is an α-diketone. Using the still moist product isolated from the solid material from Part 1, you may desire to re-crystallize this benzoin using hot 95% ethanol (you will need about 8 mL of ethanol per gram of product; determine an approximate yield from the filtration performed above. Let air be drawn through the filter before weighing to enhance the drying of your benzoin. You will use the amount specified below to do your benzil reaction. Your chemical must be dry before beginning Part 2 (re-crystallized from ethanol). Typical yield should be about 5-6 g, although some students recover less than 4 grams. Be sure to record the amount of benzoin isolated, and dry in the drying oven the benzoin not used in the benzil reaction. The product should be nearly colorless and of sufficient purity (mp 134-135oC) to use in the next reaction.
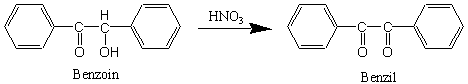
This oxidation can easily be done with a variety of mild oxidizing agents, including Fehling's solution (an alkaline cupric tartrate complex) or copper(II) sulfate in pyridine. In addition, benzoin could be oxidized by sodium dichromate, but the yield of benzil is lower because some of the benzoin is converted back into benzaldehyde following cleavage of the bond between the two oxidized carbon atoms, which is activated by the phenyl rings, producing benzoic acid as the final product. In this experiment, due to ease of use and consistent results, we will use nitric acid as the oxidizing agent.
Caution: Concentrated nitric acid is highly corrosive and causes severe burns if spilled onto your skin. Nitrogen dioxide (NO2) fumes are highly toxic and can damage the lungs due to inflammation. Do not breathe NO2 fumes, and perform this part of the experiment in the hood.
Benzil will be synthesized as follows, and must be performed in a hood:
Set up this reflux in the hood to vent NO2 gas which is produced during the reaction. With stirring, heat the reaction mixture. Begin timing the reaction when NO2 (red-brownish colored gas) are visible above the reaction mixture and gas bubbles are present on the stir bar. Reflux for at least 30 minutes, or until no more NO2 gas is being produced. Do not stop the reaction until the reaction is complete (there might still be a slight brownish color inside the flask, but as long as no more gas is being produced, the reaction can be stopped). Stop the reaction by removing the heating mantle, and letting the reaction mixture cool for about 5 minutes. Add about 75 mL of cold water to the reaction mixture, cool to room temperature, and swirl for a minute or two to coagulate the precipitated product. Collect and wash the yellow solid using vacuum filtration. Continue drawing air through the crystals on the funnel by suction for about 5 minutes to assist in drying the crystals.. The crude product (dry weight close to 4.0 grams) need not be dried but can be crystallized at once from ethanol. Dissolve the product in 10 mL of hot ethanol. To enhance precipitation of solid product from the cooled mixture, you can add some DI water drop wise until you reach "the cloud point" (the mixture goes from clear to slightly cloudy and solids start to appear), and set aside to allow crystals to form, and placing your flask on ice will enhance recovery. Record the yield, describe the crystalline form, color, and melting point of the purified, and recrystallized benzil.
Optional (at discretion of your Instructor): Test for the presence of unoxidized benzoin (only if requested by your instructor): Dissolve about 0.5 mg of crude or purified benzil in 0.5 mL of 95% ethanol or methanol and add one drop of 10% NaOH. If benzoin is present the solution soon acquires a purplish color owing to a complex of benzil with a product of autoxidation of benzoin. If no color develops in 2-3 min, and indication that the sample is free from benzoin, add a small amount of benzoin, observe the color that develops, and not that if the test tube is stoppered and shaken vigorously the color momentarily disappears; when the solution is then let stand, the color reappears.
Cleaning up: The aqueous filtrate (containing HNO3) should be neutralized with sodium carbonate, diluted with water, and flushed down the drain. Ethanol used in crystallization should be placed in the organic solvents container.
Show the correctly balanced oxidation-reduction reaction for this reaction (the "N" gets reduced, and the "C" gets oxidized: determine oxidation state for each, on the reactant side and on the product side to properly balance).
Overall reaction would be as follows.
Remember to
In this experiment, benzilic acid will be prepared by causing a rearrangement of the α-diketone benzil. (Preparation of benzil is described in Part 2 of this experiment.) The rearrangement of benzil proceeds as follows:
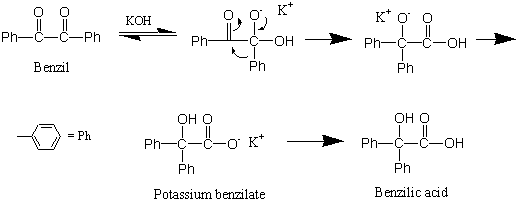
The driving force for he reaction is provided by the formation of a stable carboxylate salt (potassium benzilate). Once this salt is produced, acidification yields benzilic acid. The reaction can generally be used to convert aromatic α-hydroxyacids. Other compounds, however, also will undergo benzilic acid type of rearrangement.
Add 3.0 g of benzil and 9.0 mL of 95% ethanol to a 100-mL flask. Place a stirring bar in the flask and attach a reflux condenser. Heat the mixture with stirring until the benzil is dissolved. Add drop wise 7.5 mL of an aqueous potassium hydroxide solution1 downward through the condenser into the flask. Gently reflux the mixture for 15 minutes with stirring. After the mixture has dissolved and heated for a few minutes, the mixture will turn blue-black in color. As the reaction proceeds, the reaction product will turn brown and the solid may, or may not, be completely dissolved. At the end of the reaction, remove the flask and let it cool.
When the apparatus is cool enough to handle, remove the condenser and transfer the contents, including any solids, into a 150-mL beaker. Allow the mixture to cool to room temperature (do not rush!). When the mixture is cooled, continue the cooling in an ice-water bath for an additional 15 minutes, when crystallization should be complete. Crystallization is complete when it appears that virtually the entire mixture is solidified. If this does not occur in 15 minutes, allow the mixture to set overnight, or until complete (it is possible to store in an ice bucket or in a refrigerator, if necessary). Collect the crystals using vacuum filtration and wash the crystals thoroughly with three 15-mL portions of ice-cold 95% ethanol. The solvent should remove most of the color from the crystals.
Transfer the solid, which is mostly the potassium benzilate salt, to a 125-mL Erlenmeyer flask containing 30 mL of hot water. Stir the mixture until all the solid has dissolved or until it appears that the remaining solid will not dissolve. If solid still remains in the flask, filter the mixture through a Hirsch funnel to remove any particulate material. (If all the solid dissolved, then filtration is not required.)
With stirring, add drop wise 15 mL of 1 M HCl to the solution of potassium benzilate. The pH should be about 2; if it is higher than this add a few more drops of acid and check the pH again. Allow the mixture to cool to room temperature and then complete the cooling in an ice bath. Let the solid form in the ice bath for at least 30 min, up to about 60 min. If solid has not formed after an hour, you can store your sample until the next lab period.
Collect the benzilic acid by vacuum filtration. Wash the crystals with 30-40 mL of water to remove salts. Dry the product thoroughly in a desiccator until the next laboratory period.
Weigh the dry product and determine percent yield. Determine the melting point. Pure benzilic acid melts at 150oC. At the option of the instructor, determine the IR spectrum of the benzilic acid in KBr. Turn in the entire sample to the instructor in a labeled vial. Your grade will be based on the purity of your final product, and the amount of benzilic recovered.
___________
1The aqueous potassium hydroxide solution should be prepared for the class by dissolving 27.5 g of KOH in 60.0 mL of water. This will provide enough solution for 8 student groups, assuming little solution is wasted.
In this experiment, you will design and perform an experiment to prepare benzoic acid from benzaldehyde.
You will devise a procedure to perform this experiment and to fully characterize your product. The maximum amount of benzaldehyde you can use is 2.0 mL (2.09 g). Prior to doing this part of the experiment, you must compose an experimental protocol in complete detail, which must be approved by your instructor. Your experimental procedure but be complete, to carry out an oxidation reaction. The only things that must be done are:
Start with no more than 2.0 mL (2.09 g) of benzaldehyde. The amount of the oxidizing agent (e.g., KMnO4 or chromic acid, etc.) you add will be based on a properly balanced oxidation-reduction equation. For example, if your balanced redox equation had 3 mol benzaldehyde and 2 mol KMnO4, then determine the correct masses of each chemical based on the mass of benzaldehyde to determine the mass of KMnO4.
Use a procedure that will oxidize the benzaldehyde to benzoic acid. You must use a 250-mL round bottom flask for this oxidation reaction.
Perform a recrystallization on the benzoic acid you have prepared. Remember, recrystallization is when you take a solid chemical, and without any chemical modification, dissolve it in a solvent to supersaturate the solution, and then cool to let crystals form. Do not confuse recrystallization with the formation of a solid (e.g., treating benzoate ion with HCl to produce benzoic acid is NOT recrystallization, it is just forming an insoluble form of benzoic acid).
Authorization to perform this experiment will be given only after your protocol has been approved by your instructor (his initials on the Chemical Request Form are required). You are responsible for requesting the appropriate chemicals, and for performing the experiment safely and for characterizing your product fully. (You might have a little reprieve, since other students will likely be requesting chemicals, and if you forgot, you can still use the chemicals on the cart, even if requested by another group.)
The chemical request sheet, that must be turned in during the third day of this experiment, is available online: Chem211 Experiment 10, Part 4 Chemical Request Form
| Compound | MW | Amount | mmol | mp | bp | Density | ηD | msds |
|---|---|---|---|---|---|---|---|---|
| benzaldehyde | 106.1238 | 10.0 mL (for benzoin) | 98.5 | -26 | 179 | 1.045 | 1.5454 | msds |
| benzoin | 212.2476 | 4.8 g (for part 2) | 22.6 | 137 | 344 | msds | ||
| benzil | 210.2318 | 3.0 g (for part 3) | 14.2 | 95 | 346 - 348 | msds | ||
| benzilic acid | 228.247 | 150 - 153 | msds | |||||
| ethanol (95%) | 46.0 | 15.0 mL | -114.1 | 78.5 | 0.8 | msds | ||
| benzoic acid | 122.1232 | 122.4 | 249 | 1.08 | msds | |||
| KMnO4 | 158.0256 | 240 | 2.703 | msds | ||||
| benzaldehyde | 106.1238 | 2.0 g (for benzoic acid synthesis) | 18.8 | -26 | 179 | 1.045 | 1.5454 | msds |
| Compound | g/mol | grams or mL | 10-3 mol | oC | oC | g/mL | ηD | msds |