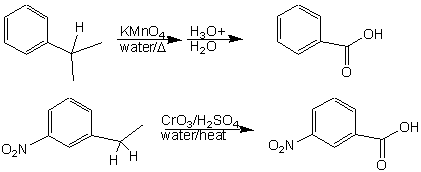
Oxidations are a very important class of reactions in organic chemistry. Most oxidations require either a heteroatom in a reduced state (O in –OH, N in –NH2, etc.) or a carbon-carbon π-bond (ozonolysis of alkenes and alkynes (and peroxide), formation of oxiranes from alkenes using peroxyacids, etc.). The oxidation of an aromatic side chain does not require any of these factors. All that is needed is a benzylic carbon with at least one hydrogen attached to it. The oxidizing agents that can be used are chromic acid (using Na2Cr2O7 or CrO3 in sulfuric acid and water) or potassium permanganate (KMnO4). The product in each case is benzoic acid or a derivative of benzoic acid (the permanganate reaction requires work up with acid, because the permanganate reaction generates a basic solution as a by product):

We will use potassium permanganate to oxidize 2-chlorotoluene (o-chlorotoluene) to 2-chlorobenzoic acid (o-chlorobenzoic acid). Permanganate solutions are less hazardous than those containing chromium compounds, which are often carcinogenic.
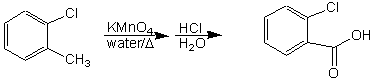
Safety: Potassium permanganate is a strong oxidizer, and is corrosive, wear gloves while handling it (it will also stain skin and clothing). 2-chlorotoluene, sodium bisulfite (hydrogen sulfite), and 2-chlorobenzoic acid are irritants–wear gloves while handling them. Concentrated hydrochloric acid is corrosive and toxic–wear gloves while handling it, and be sure to wash your gloves and your hands after handling it. Toluene, and its derivatives, is a flammable liquid, is toxic, and its vapors are narcotic–no flames will be allowed in lab, wear gloves while handling it, and avoid breathing its vapors.
In an appropriately sized round-bottomed flask (keep liquid to less 40-50% of the capacity of the reaction vessel; 250-mL flask recommended for this experiment), mix the following:
Reflux (with a few added boiling stones) the reaction mixture for about 1.5 hours. After the reflux is completed, collect the filtrate (the liquid that passes the filter) by using suction filtration in a Büchner funnel using the procedure described below (filter the hot solution, but do not wash with hot or cold water).
Note: The insoluble MnO2, which is a byproduct of this reaction, will be removed from the soluble organic product by vacuum filtration. However, if the liquid is still purple in color (indicating MnO4- ion is still present), you will need to react the remaining MnO4- ion with sodium bisulfite (NaHSO3). Remember that the reaction mixture will not be clear, but you should be able to detect the purple color. Slowly add small amounts of solid sodium bisulfite (NaHSO3) to reduce any remaining permanganate ion (how do you know if you need to do this step, and how much sodium bisulfite to add?). When no more purple color is present (the mixture will still contain the brownish-black solid suspension), you have added enough sodium bisulfite. Be careful because the reaction between sodium bisulfite and potassium permanganate is highly exothermic (heat released).
After you have added enough sodium bisulfite to remove the purple color in the reaction flask, you will collect the filtrate using vacuum filtration and a Büchner funnel. The filtrate may not be colorless, but it cannot be purple in color. After this initial filtration of the reaction contents to which sodium bisulfite has been added, the filtrate is used to isolate the benzoic acid derivative (e.g., be sure the filtrate is not purple in color). If the filtrate contains any purple color the you will need to reclaim the filtrate and add more sodium bisulfite and repeat the filtration in order to produce a clear filtrate (a slight brownish hue to the filtrate is acceptable, but it must not be purple) for isolation of the aromatic acid. Remember, at this step it is the liquid filtrate you must keep, discarding the brownish-black precipitate.
Put the clear and mostly colorless solution (remember that a slight brownish color is acceptable) in the ice bath. Acidify the mixture by adding about 2.5 mL of concentrated HCl. Add the acid drop by drop and mix by swirling the mixture. Check the pH with Litmus paper to insure that the solution is acidic. (As little as 4-5 drops may be enough to make the solution acidic, and initiate the formation of solid material. Adding more acid will not affect your results, because you cannot add too much acid.) Collect the precipitated organic product using vacuum filtration and a Büchner funnel. You can use small amounts of water to wash the solid material in the filter in order to remove any remaining solids from the crystallization container, prior to drying.
The solid product will be stored in the drying oven until the next lab period for recrystallization.
STOP, and store your solid material until the next lab period for recrystallization. (If you were not able to produce solid material during the first lab period, you can store your liquid until the next lab period.)
Recrystallize the product using toluene as solvent. Be sure to add enough toluene so that the solid dissolves at a higher temperature, but some of the solid stays undissolved at room temperature. Do not add too much toluene, as you may not be able to crystallize your product. If you believe you added too much toluene, or if o-chlorobenzoic acid crystals do not appear upon cooling the solution on ice, you will need to evaporate some of the toluene in the hood. The crystals should be suspended fully in toluene prior to filtration.
Analyze the o-chlorobenzoic acid by determining the melt temperature. Compare your observed value to the literature value. Determine a percent yield based on the limiting reagent (o-chlorotoluene).
Show a correctly balanced oxidation-reduction equation for this reaction. It is easiest to determine the oxidation state of the methyl carbon of the toluene, and the carbonyl carbon of the acid. Also, determine the oxidation states for the Mn in both the permanganate ion and the MnO2. Balance the equation based on the correct number of electrons gained, and lost.
| Compound | MW | Amount | mmol | mp | bp | Density | ηD | msds |
|---|---|---|---|---|---|---|---|---|
| potassium permanganate | 158.0256 | 3.0 g | 19.0 | 240 | 2.703 | |||
| 2-chlorotoluene | 126.5853 | 1 mL (use pipettor) | 8.5 | -35.1 | 158.97 | 1.082 | 1.5133 | |
| sodium bisulfite | 104.05587 | 1.48 | ||||||
| HCl (concentrated) | 36.4609 | -114.24 | -85.06 | |||||
| toluene | 94.1402 | used for recrystallization | -93 | 110.6 | 0.867 | 1.4969 | ||
| 2-chlorobenzoic acid | 156.5683 | 142 | 285 | 1.544 | ||||
| Compound | g/mol | grams or mL | 10-3 mol | oC | oC | g/mL | ηD | msds |
Cleanup Notes: Solid MnO2 often sticks to the glassware and is difficult to remove, even with soapy water and scrubbing. You can easily clean your glassware, however, by using 12 M HCl. In order not to waste the concentrated acid, transfer your concentrated HCl washings into a large beaker which can be used many times by other students. Clean your ground-glass joints by dipping then into the acid wash solution. To clean your flask, pour the concentrated acid into your flask and swirl. The MnO2 should be easily removed using these procedures. The acid wash solution can be re-used several times by pouring it back into the collection beaker so that it is available to other students.
Cleanup Caution: Before you use the concentrated HCl, be sure your flask is rinsed and free of any solid material. Do not leave excess water in the flask. Once your flask is clean, and free of lose residue, you can then remove the bound MnO2 which stains your flask by adding the concentrated HCl.
Go To Experiment: ChemDraw 1
2
3 4
5 6
7 8
9 10
Return to Chem211 Experiment Protocols Index
Copyright Donald L. Robertson (Date last modified: 11/14/2012)